nisin struktur
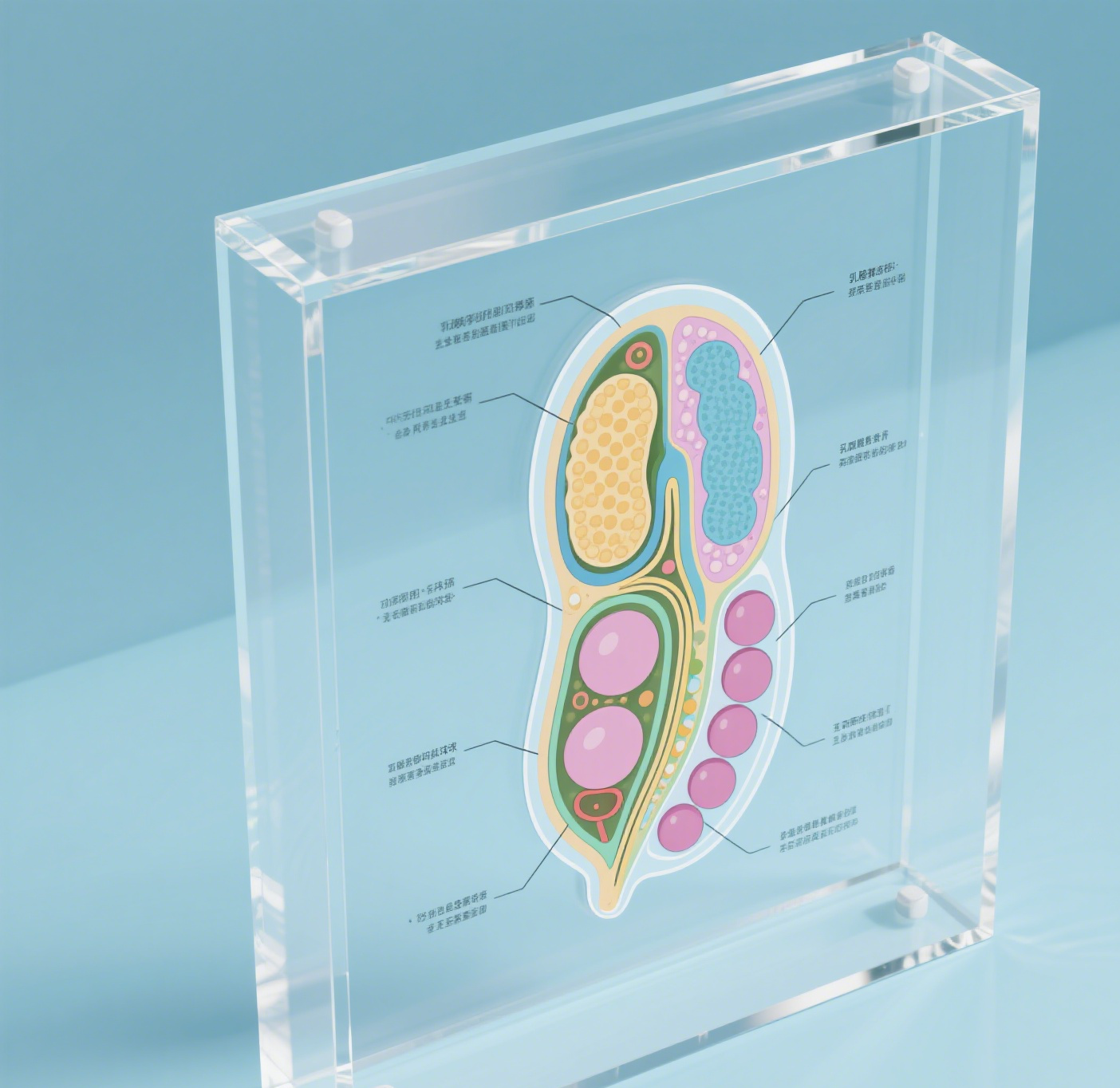
Nisin-strukturen representerer en brytningssaklig antimikrobiell peptid sammensatt av 34 aminosyresider, naturlig produsert av bestemte strener av Lactococcus lactis. Denne bemerkelsesverdige sammensetningen har en unik molekylær arkitektur kjennetegnet ved fem sammenlåste ringer, dannet gjennom spesifikke tioeterbroer og usønlige aminosyurer. Strukturen inneholder distinkte lanthionin-ringer som er avgjørende for dets antimikrobielle virkning. Disse ringene lar nisin binde effektivt til bakterielle cellevegger, opprettholder porer som til slutt fører til celledd. Peptidets amfifil natur, som kombinerer både hydrofil og hydrofob regioner, lar det fungere effektivt i ulike miljøer. Dets molekylvægt på omtrent 3 354 dalton gjør det særlig effektivt mot gram-positiv bakterier, mens dens stabilitet i sure vilkår forsterker dets praktiske anvendelser. Strukturens bemerkelsesverdige fleksibilitet lar det beholde sine antimikrobielle egenskaper over ulike temperaturintervaller og pH-verdier, noe som gjør det uerstattelig i matbevaring og farmasøytiske anvendelser. Videre viser nisin-strukturen fremragende biodegradabilitet og mangler toksisitet mot mennesker, hvilket gjør det et miljøvennlig og trygt valg for ulike industrielle anvendelser.